Why Ice Cube Is Not Clear
And the same question concerns ice balls too.
And some spoiler: ICE CUBES/BALLS CAN BE FROZEN TO BE CLEAR. And it can be done at home.
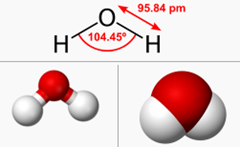
The water molecule is formed from one oxygen atom and two hydrogen atoms. => H2O
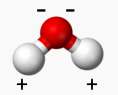
The molecules are tiny magnets which can attract to each other and repel. We’ll call those forces hydrogen bonds.
Liquid water has a disorderly arrangement of molecules because they move too fast. They are called to have much of kinetic energy. And they can’t attract or repel each other to the extent to stop each other’s movements.
Here is another view of the water molecule.
The oxygen atom is located at the center of a tetrahedron (just a scientific name for a “triangular pyramid”) while the two hydrogen atoms are located at two vertices (another sci word for a “corner”).
In the other two vertices there are lone pairs of electrons. You don’t see them in the gif image.
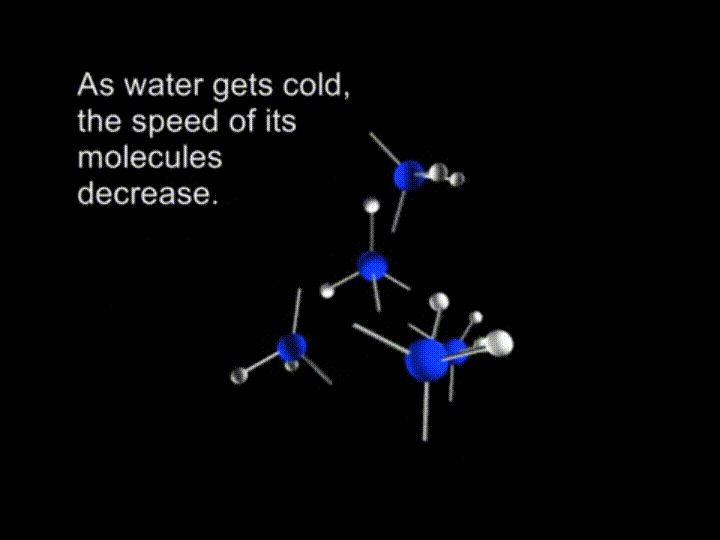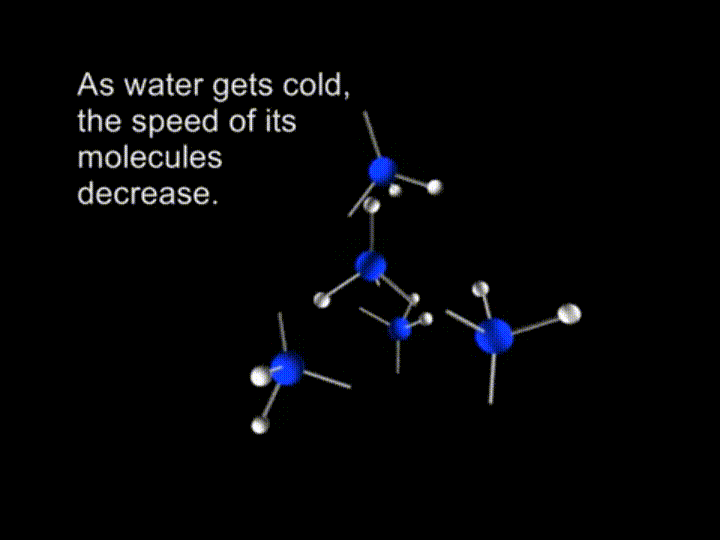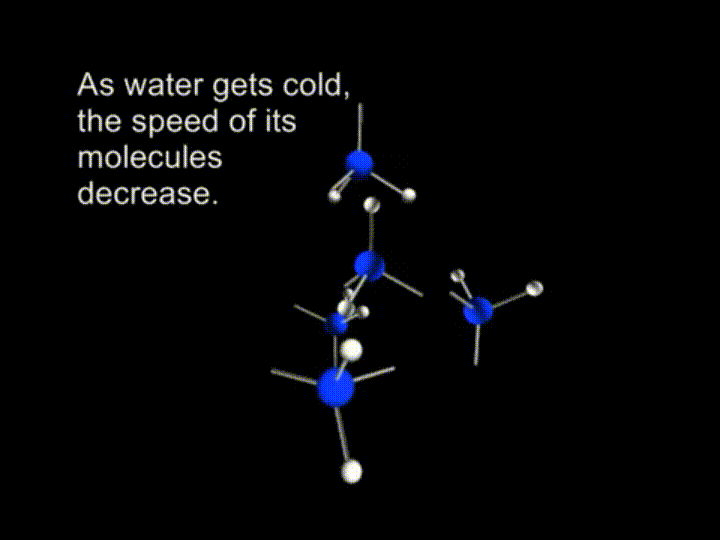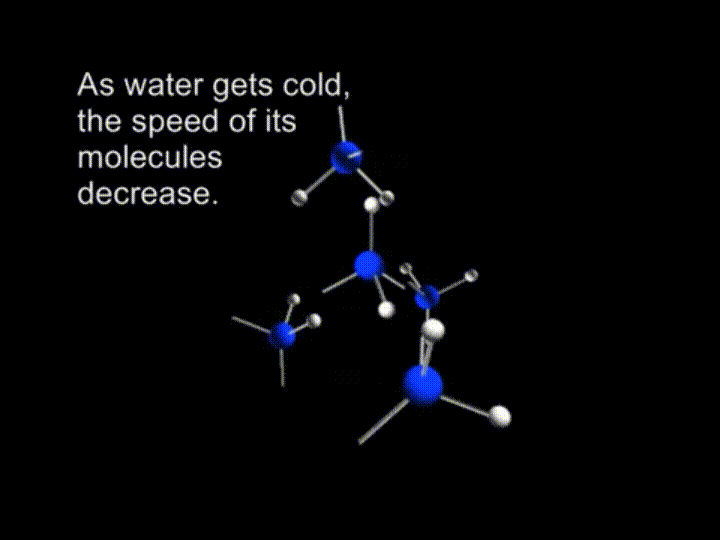
So, we have a free motion of the molecules in water. The average kinetic energy of the liquid water is big enough to break the hydrogen bonds. As water gets cold, the speed of its molecules decrease.
And finally the molecules are slowing down to the extent when retained by the hydrogen bonds.
In ice crystals each molecule is surrounded by four neighboring water molecules joined together by hydrogen bonds.
And at this point every single water molecule is surrounded by other four. They form another tetrahedron with a one molecule sitting in the center of pyramid and other four sitting in the corners.
We have a perfect clear ice at this point. If you are an observant student you could see that new structure is less dense. This is an explanation, by the way, why ice is lighter then water. The ice is less dense than liquid water and floats in it.
When the things go wrong with making ice cubes at home
That was good in theory. But try to freeze some water into ice and you’ll end with cloudy whitish ice. Why is that?
Simply speaking it becomes cloudy because of impurities and air bubbles trapped into the structure of ice. You’ll not get such geometrically ordered arrangement of the water molecules as in the last gif. Ordered structure will by intermingled with impurities and air.
Even if you have a distilled water it doesn’t help because of air bubbles.
Air bubbles should be driven out. Don’t boil water – that won’t help too.
How to make a perfect clear ice
If we just could make the water molecules get into the perfect arrangement. And we can!
You saw that water molecules slow down their motion gradually. And as far as we have a volume of water we’ll have the points of nucleation (crystallization) in different places in this volume. In those points the ordered arrangement happens first. After that the air bubbles just destined to be trapped between them.
But if we are smart we can direct the heat deprivation (or make a directional freezing). We have to ensure that points of nucleation show up at one side and the arrangement of molecules is happening gradually across the volume of water.
In that case water molecules will not allow “insiders” to pass into their arrangement.
Not only air bubbles but even impurities are driven out in that way.
Stay tuned to know how to make clear ice at home in a step-by-step guide...










No comments
Post a Comment